Why does pharma need a fresh approach to clinical trials?
The first factor to consider is the associated costs.
Studies show that clinical trials of new drugs last nine years on average and cost around $1.3 billion to execute. The cost of failed clinical trials, meanwhile, ranges between $800 million and $1.4 billion. And the fact that 90% of all drugs end up failing clinical trials only complicates the matter.
Another factor is the practicalities.
In traditional clinical trials, doctors and researchers manually look for participants, and patients have to be physically present to enroll and undergo evaluation. The treatment is also carried out on site through scheduled visits. This approach is slow and inflexible, and it doesn’t allow for integrating and processing data from hospitals, research centers, private practices, and patients’ homes. Researchers struggle with participant recruitment and request patients to visit trial sites for systematic condition reviews and monitoring, which could increase the chances of patient dropout.
Let us investigate how artificial intelligence and its subtypes can help resolve these issues.
How can AI modernize clinical trials?
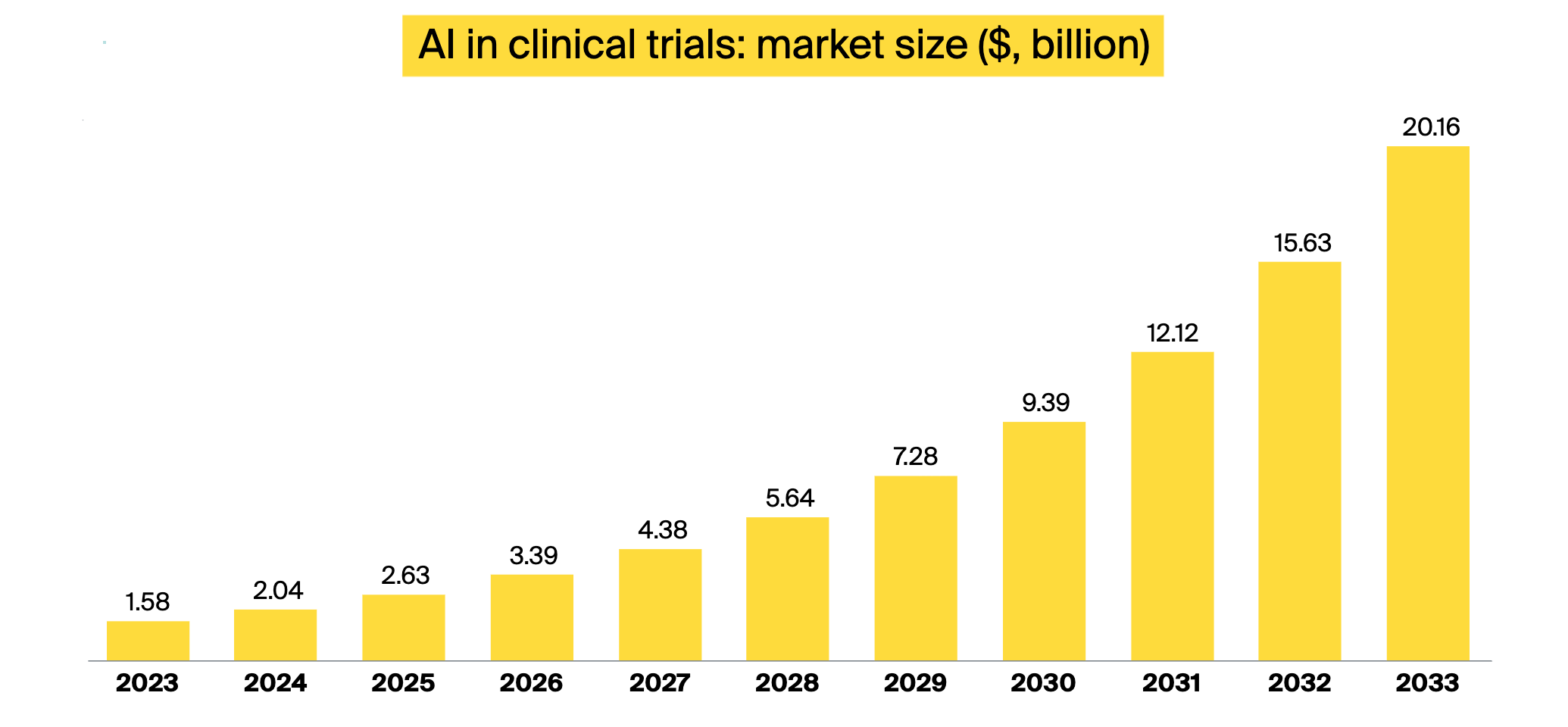
The global AI in clinical trials market is on the rise. It was valued at $1.58 billion in 2023, and it’s expected to skyrocket to $20.16 billion by 2033, growing at a CAGR of 29%.
AI can integrate data from multiple sources, including electronic health records (EHRs), research papers, past clinical trials, and specialized medical case studies. It can also handle the continuous stream of data from personal medical devices. AI models analyze the data to help make sense of it all, source the right patients for each trial, predict how patients can react to the proposed remedies, and more.
Besides making clinical trials more data-oriented, AI can also cut down on costs and time needed to develop a new drug. For example, research shows that using AI in clinical trials for patient recruitment can reduce trial costs by 70% while shortening trial timelines by 40%. Another study indicates that incorporating AI into the clinical research phase could save the pharmaceutical industry $28 billion per year.
The table below depicts how AI can streamline every aspect of clinical trials.
| Artificial intelligence and analytics | AI-powered wearables, connected devices | Generative AI | |
|---|---|---|---|
| Clinical trial design |
Selecting trial protocols, location, the optimal number of participants, and any other parameters |
|
|
| Participant recruitment |
|
Evaluating potential candidates against trials’ inclusion and exclusion criteria remotely by measuring different parameters |
Screening candidate eligibility by reviewing their medical records and analyzing their genetic makeup |
| Participant monitoring and engagement |
|
|
|
| Augmenting trials |
Creating virtual patients and using them at early stages of trials |
|
|
| Trial data analysis and documentation |
Analyzing clinical trial data |
|
|
Artificial intelligence in clinical trials: Top 9 applications
Below are nine key applications of AI in clinical trials. Here is what the technology can do.
-
Designing clinical trials
-
Facilitating participant recruitment
-
Assessing participant eligibility
-
Managing the consent process
-
Monitoring participant adherence
-
Aiding in clinical trial data collection and analysis
-
Boosting personalized patient engagement
-
Generating trial documentation drafts
-
Augmenting trials with virtual patients
1. AI helps design clinical trials
To design a clinical study, pharmaceutical companies need to look through vast amounts of data, approximately 80% of which is unstructured and hard to analyze. AI for clinical trials can help aggregate and process all this data and find useful patterns. For example, the technology can derive regulatory protocols, strategies, and patient enrollment models that suit the country of the trial.
AI can also find ways to decrease the number of trial participants. A research team from Nihon University, Japan, used an AI-powered genetic algorithm to optimize a blood sampling schedule for a pediatric study. The algorithm adjusted the study parameters to reduce the number of subjects donating blood from 15 to seven without compromising the results.
AI generates trial strategies
Generative AI (Gen AI), which is a subtype of AI that produces original content resembling human creation, can draft trial strategies based on input from the scientists. Domain-specific large language models (LLMs), such as ClinicalBERT, can simulate trial scenarios based on different strategies. A trial team can give feedback on a proposed approach, and the model will generate another version, taking these suggestions into account.
You can learn more about Gen AI pros and cons and how it differs from artificial intelligence on our blog. Also, check out our post on how Gen AI impacts the pharmaceutical sector.
2. AI facilitates participant recruitment in clinical trials
Traditionally, patients can learn about relevant trials from their physician or search information in a corresponding database, like the national US registry of clinical studies. Many willing and eligible participants are simply not aware of the opportunities.
Enhancing clinical trials with AI allows for sifting through patient data, such as EHRs and medical images, to compare patient characteristics to the study’s eligibility criteria, identify the right individuals for a particular trial, and invite them in. AI is powerful enough to select a homogeneous set of participants, which is challenging with the conventional methods.
An AI startup, Deep Lens, uses its vast database of oncology studies to recruit patients for trials. The startup can match people newly diagnosed with cancer and expedite their enrollment in trials. In a similar vein, 23andMe, a personal genetics company based in California, suggests clinical studies to its clients based on their genetic makeup.
3. AI assesses participant eligibility for clinical trials
Candidate participants need to go through evaluations to ensure they meet the inclusion criteria, which demands their physical presence. And depending on their location and job flexibility, they may not be able to visit the trial’s facilities during the designated time slot. Using wearable technology, patients can conduct some evaluations at home. This data, once collected, can be run through AI algorithms, giving insights into the patients’ well-being.
For example, TytoCare, a medical startup, offers connected examination tools and underlying mobile apps that enable patients to capture measurements from their lungs, heart, skin, throat, etc. and send them to clinicians.
AI can also study medical records to assess a candidate’s eligibility. Researchers from Mass General Brigham conducted a study where a Gen AI model screened heart failure patients for clinical trial fitness based on their medical records. The model identified suitable patients with an accuracy of 97.9%.
4. AI assists in consent management
Researchers organizing a clinical trial need to obtain informed consent from every participant. This helps ensure that everyone is aware of the trial’s scope and the associated risks.
Handling the informed consent forms (ICF) is a tedious administrative process that might need revision if a trial protocol changes. AI in clinical trials can help partially automate this process. For example, DataArt developed an AI model that can automatically extract relevant details from trial documents and fill this information in the corresponding ICF fields. Afterwards, the forms are sent to the participants to sign them.
5. AI monitors participant adherence
Medication non-adherence is rather common. Studies indicate that 50% of Americans fail to take their long-term chronic medication as instructed.
In clinical trials, the process of manually tracking medication adherence is prone to error as it relies on patients’ memory. Doctors also often use unreliable recording systems, such as pen and paper.
Deploying wearables together with clinical trial AI allows researchers to monitor patients’ actions through automated data capture instead of waiting for the patients’ manual reports. For instance, AiCure, one of the prominent AI clinical trial companies, developed an interactive medical assistant that can spot patients at risks of non-adherence. This technology also allows patients to record a video of themselves swallowing a pill as proof that they actually did so. The assistant can identify the patient and the pill, confirming medication intake to the responsible doctor.
6. AI aids in clinical trial data collection and analysis
Clinical trials produce and consume massive amounts of data. Every participant would generate excessive information, such as adherence data, vital signs, and any other intermediate feedback. Especially now that clinicians can monitor patients in their homes in real time with the help of medical IoT devices and the Internet of Bodies. This means processing large amounts of data daily. AI can take over this task and spot and report any deterioration in patients’ conditions, ensuring patient well-being and minimizing dropouts.
Clinical trial software providers incorporate AI into their solutions to enhance data analysis. Clario, a Philadelphia-based healthcare research tech provider, announced integrating over 30 different AI models in its clinical trial management platforms to support collecting and analyzing a wide range of digital data while maintaining participants’ privacy.
7. AI boosts personalized patient engagement
Using AI in clinical trials can help engage participants. AI systems can send reminders and notifications based on real-time patient data analysis results and trial protocols. Generative AI-powered chatbots can initiate personalized conversations with patients and respond to their queries about reimbursement, care options, and more.
McKinsey reports that deploying Gen AI to promptly assist trial participants can reduce the drop-off rate by 5%–10%.
8. AI generates clinical trial documentation drafts
Artificial intelligence can help research teams compose trial documents. Intelligent algorithms can create initial drafts that researchers will review and modify. For instance, India-based Clinion offers AI-powered clinical trial software that includes a report generation feature. The company claims that this product can generate 60%–70% of the documents while reducing errors by up to 70%.
9. AI augments clinical trials with virtual patients
Clinical researchers can test novel drugs on virtual patients in the early stages of trials as an additional safety measure before moving to humans. But what is a virtual patient exactly?
A virtual patient is a digital twin of a real person. Researchers feed an AI model with a detailed patient profile, including their medical history, genetics, etc., to have a comprehensive view of an individual and accurately predict how they will react to drug compounds.
A French multinational pharma company, Sanofi, relies on this technology. The company integrates virtual patient details along with the available information on a particular disease, such as its pathophysiology, biology, etc., into a computational framework to predict how the disease will progress under different treatment options.
A few words about the challenges of using AI in clinical trials
If you are looking to deploy artificial intelligence in clinical trials, be prepared for the issues described below.
-
Lack of interoperability in medical data. Despite the efforts invested in unifying medical data, there are still multiple healthcare IT standards, and healthcare data interoperability remains a challenge. This makes it hard to integrate patient information from medical organizations that use different EHR software, not to mention that some doctors still rely on handwritten notes.
-
The presence of AI bias. AI models can develop bias if the training dataset is not representative of the actual population, as the generalizability of the model depends on the diversity of data that the algorithms consume during training. For example, improperly trained models can skew site suggestions for clinical trials or perform poorly on patients of color.
-
Lack of quality training datasets. It’s challenging to obtain comprehensive datasets for each disease, especially for the rare ones. And it doesn’t get easier if we research patient data, as companies need to be careful with privacy and security. When the training data is scarce, you can turn to Gen AI to generate synthetic but realistic datasets. MOSTLY AI is one of the vendors who can produce synthetic data for healthcare and pharma.
-
Hallucination. If you use generative AI in your clinical trials, keep in mind that this technology can produce plausible but incorrect output. Make sure you implement some verification techniques like the human in the loop approach to spot hallucinations before they influence the trial’s outcome.
To sum up
Artificial intelligence has an immense potential to streamline clinical trials. From generating trial strategies to recruiting participants and drafting documentation, there’s no task AI can’t assist you with. But to successfully implement the technology, you will most likely need a reliable AI development partner.
ITRex has a vast experience in AI and its subtypes, including generative AI development. Our diverse team also includes IoT consultants if you are looking to support your clinical trial with connected wearable and stationary devices and want to collect and analyze participants’ data in real time. And speaking of data, there will be a lot of it. Our data consultants can help you with management and governance strategies.
Get in touch, and we can start with an AI proof of concept, which enables you to experiment with artificial intelligence in clinical trials without committing to a full project.