Four ways in which technology streamlines compliance in pharma
1. Overcoming silos
Cloud-based data management, data warehousing, big data analytics, and other technologies can help pharma companies consolidate data that was previously stored across siloed systems.
Consolidated data storage allows pharmaceutical companies to gain a 360-degree view of their data and detect compliance issues early on.
One example of a company leveraging modern tech to improve pharma compliance by boosting data management is Pfizer. The leading pharma corporation relies on an electronic document management system that consolidates clinical trial data from multiple sources for a single, unified view. It conveniently tracks the progress of clinical trials, identifies trends and patterns, and ensures that trial data is accurate and up-to-date.
Another example comes from Johnson & Johnson, which uses a cloud-based data management system that integrates all enterprise data coming from different sources. The solution facilitates the company’s efforts in achieving compliance in pharma by maintaining accurate and up-to-date records of all activities related to drug development, testing, and distribution. This includes tracking all stages of drug development, including research, clinical trials, regulatory approval, and post-market surveillance.
2. Enabling advanced reporting
By relying on new-gen reporting tools, pharmaceutical companies can now generate customized reports of any complexity (in real time as well). This helps them gain deeper insight into their data, identify potential pharma compliance risks, and take proactive measures to address them.
Moreover, technology tools can help pharma companies avoid duplicate or contradictory reports across different departments or regions, ensuring that the data submitted to regulatory bodies is consistent and accurate.
The Jonhson & Johnson’s data management system mentioned above is a worthy example of how technology facilitates pharma compliance through efficient adverse event reporting. The Johnson & Johnson’s solution allows the company to efficiently track and report adverse events and side effects, ensuring that all necessary information is collected and submitted to regulatory bodies in a timely manner.
Another global pharmaceutical company, Sanofi, uses advanced reporting to facilitate their efforts in achieving compliance in pharma. Technology tools allow the company to generate customizable reports that meet the specific requirements of each regulatory body they deal with. All reports are generated automatically, saving time and minimizing the potential for human error.
3. Facilitating business processes
Another use case of technology in facilitating pharma compliance is improving business processes. Specifically, technology can help streamline and even automate the following tasks:
-
Administration, tax, and finance: accounts payable, payment processing, bank reconciliation, and tax reporting
-
Purchasing: expense analysis, expense report management, procurement data management, supplier relationship management, and contract management
-
Supply chain: demand forecasting, inventory and assets management
-
Human resources: travel and expense management, employee data management, time record validation, and others.
Many pharma companies across the globe are leveraging the power of tech to facilitate and automate mundane workflows. For example, Novo Nordisk, a multinational pharmaceutical company headquartered in Denmark, uses technology to automate indirect tax compliance management across more than 50 countries the company operates in, which significantly improves their pharma compliance management programs.
The solution is integrated with the company’s ERP system, which helps quickly identify the potential areas of non-compliance. It also provides real-time visibility into the company’s tax position, enabling Novo Nordisk’s executives to make more informed decisions and optimize their tax strategy.
Another example of relying on business process optimization for maintaining compliance in pharma comes from AstraZeneca. The global pharmaceutical company has adopted a vendor management system that allows them to effectively manage their global vendor relationships, monitor vendor performance, and keep an eye on the vendors’ compliance with regulatory requirements. The system maintains a central database of vendors and their associated compliance documentation, including qualifications, certifications, and financial records.
4. Speeding up drug development
The growing use of technology helps pharma companies reduce the time and cost associated with bringing new drugs to the market.
The ways in which modern tech allows speeding up drug development — while helping maintain pharma compliance — are many:
-
Advanced data analytics and machine learning tools can accelerate drug discovery and identification of potential drug candidates
-
Using computer simulations and digital twins can help predict drug behavior before it is tested live
-
Electronic health records and other patient data management systems can help identify eligible patients for clinical trials and speed up recruitment
-
Wearable devices and monitoring technology can provide real-time data on patients in clinical trials, reducing the time and cost of collecting data
-
Internet of Things-powered manufacturing equipment can accelerate the production of drug prototypes while guarding drug quality
-
Robotic process automation (RPA) tools can automate repetitive tasks in drug development, freeing up researchers’ time
-
Cloud computing technologies can provide scalable and cost-effective storage and processing of large amounts of data, providing for faster analysis and decision-making.
One example of a company that has already discovered the value of tech in speeding up drug development is GSK. The British pharmaceutical and biotechnology company uses artificial intelligence to test drug compounds more quickly, efficiently, and accurately. The company also relies on AI to identify potential drug candidates, significantly reducing the time it takes to move them to clinical trials.
Another example of a company using tech to improve pharma compliance comes from Novartis. The Swiss pharma corporation uses AI to analyze vast amounts of data from its drug discovery and development programs. Specifically, they apply AI to analyze patient data, preclinical data, and genetic information to identify potential drug targets and optimize drug candidates.
Novartis also uses AI to design new drug compounds with specific properties and to predict how those compounds will interact with biological targets.
Barriers to using technology for pharma compliance management
While technology can be extremely helpful in achieving compliance in pharma, there are several barriers that companies must overcome in order to tap into the full value of tech.
One of the most significant ones is the lack of digital maturity within the sector. Digital maturity refers to the extent to which an organization is relying on digital tools and technology. Many pharma companies still depend on legacy systems, which can be difficult to integrate with modern technology solutions.
A survey conducted by DT Сonsulting among global pharma leadership highlights the following reasons for the low digital maturity in the sector:
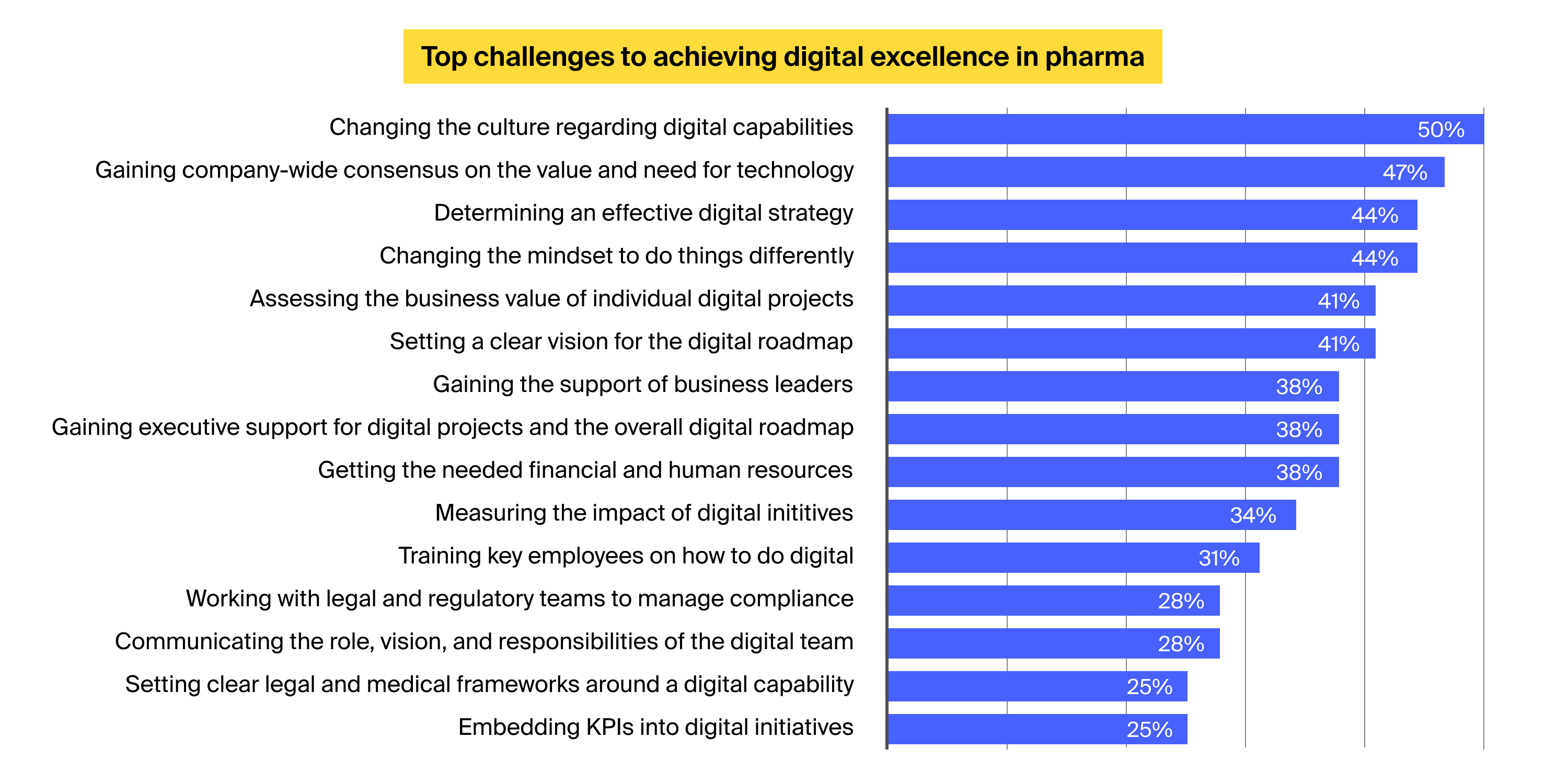
Improving the sector’s digital maturity and streamlining pharma compliance efforts calls for sector-wide change and requires addressing the following bottlenecks:
-
The perceived risk associated with adopting new technology
The pharma industry is highly regulated, and any change to processes or systems must be carefully evaluated and tested to ensure compliance with regulatory requirements. This cautious approach can lead to slow decision-making, resulting in delays in the adoption of new technology.
Solution: To minimize the risks associated with adopting technology solutions, we advise you to collaborate with regulatory authorities and engage with them early in the transformation process in order to ensure pharmaceutical compliance and get a buy-in.
Another solution to consider is starting your digital transformation initiative with a pilot study. Conducted in a controlled environment, it could help you test new technologies, as well as demonstrate their compliance and safety.
-
The lack of technology standardization in the sector
Many pharma companies have grown through mergers and acquisitions, resulting in a patchwork of legacy systems and processes that are often difficult to integrate. This can make it challenging to implement new technology solutions, as they may not be compatible with the existing systems.
Solution: To overcome this issue, we recommend running a thorough assessment of current systems and processes to identify potential bottlenecks, prioritizing the implementation of technology solutions that are compatible with existing systems, and resorting to iterative implementation to ensure seamless integration of all technology components.
-
The lack of interoperability
The pharma sector is highly dependent on data. Data silos and the lack of interoperability can hinder the adoption of technology and pose a challenge to maintaining compliance in pharma. Different departments within a pharma company may use different systems or even different versions of the same system, making it challenging to consolidate data and gain a holistic view of the organization’s operations.
Solution: To ensure data integrity and software interoperability, consider developing a comprehensive data strategy that would define data governance, ownership, and standardization policies. It also pays off to conduct regular data audits to identify data silos and inconsistencies.
-
The lack of digital skills and expertise within the industry
The pharma sector has traditionally been slow to embrace new technology and may lack the necessary resources, skills, and expertise to implement digital solutions.
Solution: To close the expertise gap, pharmaceutical companies can either invest in recruiting experienced professionals who can lead the implementation of the new technology or turn to external consultants with the expertise developing solutions for pharma to provide strategic and technology leadership.
On a final note
Even though historically, the pharmaceutical sector has been rather slow in adopting technology for better pharma compliance management, the outlook is changing. Regulatory authorities themselves prompt the industry to jump on the innovation bandwagon.
In the US, the FDA created the emerging technology program that encourages innovative approaches to pharmaceutical product design and manufacturing. Similarly, the Process Analytical Technologies (PAT) team established by the EMA and the MHRA (Medicines and Healthcare products Regulatory Agency) leads innovation in the EU and UK.
The success stories of early adopters prove: technology solutions do make it easier to achieve and maintain compliance in pharma. Still, to reap the benefits, it is crucial to approach the implementation of novel solutions cautiously and with the potential challenges in mind.
To learn more about how technology solutions can help your company achieve and maintain compliance, get in touch with our technology consultants.













